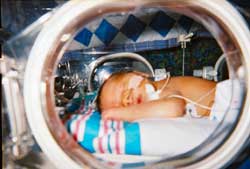
 A neonatal intensive care unit, usually shortened NICU (pronounced "Nickyoo") and also called a newborn intensive care unit, and special care baby unit (SCBU - pronounced "Skiboo"), is a unit of a hospital specialising in the care of ill or premature newborn infants. NICUs were developed in the 1950s and 1960s by paediatricians to provide better temperature support, isolation from infection risk, specialised feeding, and access to specialised equipment and resources.
A neonatal intensive care unit, usually shortened NICU (pronounced "Nickyoo") and also called a newborn intensive care unit, and special care baby unit (SCBU - pronounced "Skiboo"), is a unit of a hospital specialising in the care of ill or premature newborn infants. NICUs were developed in the 1950s and 1960s by paediatricians to provide better temperature support, isolation from infection risk, specialised feeding, and access to specialised equipment and resources.
Infants are cared for in incubators or "open warmers." Some low birth weight infants need respiratory support ranging from extra oxygen (by head hood or nasal cannula) to continuous positive airway pressure (CPAP) or mechanical ventilation. Public access is limited, and staff and visitors are required to take precautions to reduce transmission of infection.
By the 1970s SCBU's were an established part of hospitals in the developed world. In Britain, some early units ran community programmes, sending experienced nurses to help care for premature babies at home. But increasingly technological monitoring and therapy meant special care for babies became hospital-based.
By the 1980s, over 90% of births took place in hospital anyway. The emergency dash from home to SCBU with baby in a transport incubator had become a thing of the past, though transport incubators were still needed. Specialist equipment and expertise were not available at every hospital, and strong arguments were made for large, centralised SCBUs. On the downside was the long travelling time for frail babies and for parents. A 1979 study showed that 20% of babies in SCBUs for up to a week were never visited by either parent. Centralised or not, by the 1980s few questioned the role of SCBUs in saving babies. Around 80% of babies born weighing under 1.5kg now survived, compared to around 40% in the 1960s. From 1982 in Britain pædiatricians could train and qualify in the sub-speciality of neonatal medicine. Not only careful nursing, but also new techniques and instruments now played a major role. As in adult intensive care units, the use of monitoring and life support systems became routine. These needed special modification for small babies, whose bodies were tiny and often immature. Adult ventilators, for example, could damage babies lungs and gentler techniques with smaller pressure changes were devised.
Incubators for control of temperature and environment:
Temperature regulation in newborns.
 One of the most important elements in a newborn's survival is the infants temperature regulation. Mammals have the advantage of being homeotherms, meaning that they are able to produce heat, allowing us to maintain a constant body temperature. However, homeothermy may be overwhelmed in extremes of cold or heat. The newborn baby has all the capabilities of a mature homeotherm, but the range of environmental temperature over which an infant can operate successfully is severely restricted.
One of the most important elements in a newborn's survival is the infants temperature regulation. Mammals have the advantage of being homeotherms, meaning that they are able to produce heat, allowing us to maintain a constant body temperature. However, homeothermy may be overwhelmed in extremes of cold or heat. The newborn baby has all the capabilities of a mature homeotherm, but the range of environmental temperature over which an infant can operate successfully is severely restricted.
The infant has several disadvantages in terms of thermal regulation. An infant has a relatively large surface area, poor thermal insulation, and a small amount of mass to act as a heat sink. The newborn has little ability to conserve heat by changing posture and no ability to adjust their own clothing in a response to thermal stress.
Responses may also be hindered by illness or adverse conditions such as hypoxia (below normal levels of oxygen). Heat exchange between the environment and the infant is like any physical object and its environment. Heat is exchanged by conduction, convection, evaporation, and radiation. Heat exchange by conduction is relatively small. Conduction depends on the thermal conductivity of a substance in contact with the body. Since babies are usually laid on a mattress, which has a relatively low thermal conductivity, the heat loss from the baby to the mattress is relatively small. Heat loss from the infant by convection is dependent upon air speed and air temperature.
Evaporative loss depends upon air speed and the absolute humidity of the air. If a baby is clothed or nursed in a regular warm air incubator of moderate humidity, evaporative heat loss is only a small fraction of the total heat lost by the infant. However, if an immature baby with thin skin is nursed under a radiant overhead heater in a normal nursery environment, evaporation is a major factor for heat loss. Radiant heat loss is slightly more complex than the rest. It is dependent on the surface area and geometry, the surface temperature of the body, as well as the temperature of the receiving surface area.
The infant's body responds differently to hot and cold temperatures. In the case of hotter environmental temperatures, the infant's body produces sweat through the sweat glands. The basal metabolic rate increases, causing the body temperature to rise. The risks of hyperthermia are great and should be attended to immediately. Serious overheating can cause heatstroke or death, and lesser degrees of stress can cause cerebral damage due to hypernatremic dehydration. Babies born more than 8 weeks before term have virtually no ability to sweat. Even in a baby born only 3 weeks early, sweating is severely limited and confined to the head and face. Sweat production matures relatively quickly in the pre-term baby after delivery, allowing the baby to be placed in a regular crib.
In the case of cold environmental temperatures, the infant may produce heat by shivering and other muscular activity. Cold stress is subtler in its consequences but must be attended to. Newborns may also be placed in a neonatal incubator.
Incubator
A neonatal incubator is a device consisting of a rigid box-like enclosure in which an infant may be kept in a controlled environment for medical care. The device may include an AC-powered heater, a fan to circulate the warmed air, a container for water to add humidity, a control valve through which oxygen may be added, and access ports for nursing care. It may also contain a servocontrol to help regulate incubator air temperature. The servocontrol uses a temperature sensing thermistor, which is taped to the child's abdomen.
In infants born before 31 weeks gestation, evaporative water loss is the single most important channel of heat loss. This is due to inadequate keratinisation of the skin, which allows a high permeability of water to the skin. The permeability drops rapidly in the first 7 to 10 days after birth unless the skin becomes traumatized or secondarily infected. In that 7 to 10 day period, the absolute humidity must be monitored so that evaporative heat loss is kept to a minimum as well as water loss through the skin.
Premature babies are not always put in incubators. If a baby is in danger of going into respiratory arrest or other significant problems, they are put in an overhead radiant cradle so that they are easily accessible to nurses and doctors. The radiation from overhead puts the heat back into the baby while the baby is losing heat by other means. Heat losses and gains are difficult to monitor. The only way to monitor the baby's temperature is with a thermistor and servo controlled heating unit. The overhead radiator can account for the heat lost by other means, but cannot account for the water lost through the skin, which is critical to maintain for the first 7 to 10 days after birth to prevent dehydration.
There have been significant advances in thermoregulation since the 1960s. These advances have reduced mortality in small babies by 25%. Although this is a great accomplishment, research continues so that the mortality in small babies is reduced even more.
Design and performance
The design and performance features of the neonatal incubator and transport incubators provide the basis for understanding the intended uses and capabilities of the device. Design features address the intended uses of the device to meet the need of the user and the patient, while the performance features ensure that the devices are safe and effective when use in accordance with the directions. The features listed in this section are important in determining whether the neonatal incubator or neonatal transport incubator is substantially equivalent to a legally marketed device. A complete discussion of many of these features can be found in IEC standards documents.
Standards:
- IEC 601 2 19 Amendment I Medical Electrical Equipment - Part 2: Particular Requirements for Safety of Baby Incubators;
- IEC 601-2-20 Medical Electrical Equipment - Part 2: Particular Requirements for Safety of Transport Incubators;
- ISO 10993-1: 1992 Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing
The following topics are discussed:
- Materials of construction
- Engineering diagrams
- Mode of operations
- Power source
- Heating and cooling mechanism
- Air controlled versus baby controlled
- Supplementary gas connectors
- Functionality
- Physical durability and robustness
- Environmental conditions for proper operations
- Performance
- Temperature control
- Accuracy
- Rise Time
- Variability
- Undershoot/Overshoot
- Air Flow
- Velocity
- Flow rates
- Supplementary Gas control
- Connectors
- Mixing/Concentrations
- Safety features
- Weight capacity
- Electrical
- Restraints
- Alarms
- Fire protection
- Sensors
- Lockout
Thermo-neutrality
Thermo-neutrality is one of the major environmental factors affecting a premature or low-birth-weight neonate inside an incubator. Severe temperature differences inside an incubator lead to neonate heat loss, hypothermia and apnoea, which are closely related to air flow and air velocity. (An anatomically correct neonate model is designed using a three-dimensional laser scanner system and a rapid prototyping machine.) Flow visualisations demonstrate that large-scale rotating airflow is produced inside the chamber, and a number of small, stationary eddies are found in regions between the air inlet and the neonate. Hot-wire measurements show that air velocities along the long inlets are not uniform. Computational fluid dynamics show relatively uniform temperatures of about 34 degrees C on the neonate's anterior aspect and the highest temperature of 36.1 degrees C at the right armpit and the crotch. Flow fields from airflow visualisations, hot-wire measurements and computational fluid dynamics are very similar, both qualitatively and quantitatively. The small eddies produced between the neonate and the mattress could interfere with convective and evaporative heat transfers from the neonate. Therefore it is important to eliminate eddies around the neonate in the design of neonatal incubators.
Biocompatibility of materials
Biocompatibility testing is required for all parts of the device that have direct or indirect contact the patient and is performed to determine the potential toxicity that can result from contact of the component materials of the device with the patient's body. The materials used in the construction of the device should not, either directly or indirectly through the release of their material constituents, produce unreasonable risk of adverse local or systemic effects; be carcinogenic; or cause adverse reproductive and developmental effects.
The evaluation of any new device intended for human use requires data from systematic testing to ensure that the benefits provided by the final product will exceed any potential risks produced by device materials. Biocompatibility testing is indicated when a "new" or non-conventional material or chemical component is incorporated into a device and there is no known appropriate predicate use or for which the safety or effectiveness of the resulting formulation is in question. These materials or chemical components include plastics, metals, colorants, plasticizers, germicides, and chemical or other treatments of the device or device components.
Biocompatibility testing should be performed on the finished product, using test conditions simulating as closely as possible actual patient use. Materials and chemical components that have already been incorporated in legally marketed devices with similar conditions of use, or have a demonstrated history of safety and effectiveness may not require biocompatibility testing; however, the biocompatibility of these materials and chemical components should be fully discussed to support the lack of testing. (Refer to ISO-10993-1:1992, Part I "Biological Evaluation of Medical Devices, Evaluation and Testing", and the FDA-modified Matrix to identify the types of biocompatibility testing that should be considered in evaluating the safety-in-use of medical devices and materials.
The ISO Standard, Part 1, uses an approach to test selection that is very similar to the Tripartite Guidance. It also uses a tabular format (matrix) for laying out the test requirements based on the various factors discussed above. The matrix consists of two tables, "Initial Evaluation Tests for Consideration" and "Supplementary Evaluation Tests for Consideration."
Labelling
It is important to the user that the labelling for the neonatal incubator and neonatal transport incubator bear clear, accurate, and complete information for use concerning any relevant indications for use, conditions and limitations of use, hazards, contraindications, and precautions in their use.
Sources:
http://www.fda.gov/RegulatoryInformation/Guidances/ucm073955.htm#SAFETY
http://www.iec.ch/
http://en.wikipedia.org/wiki/Neonatal_intensive_care_unit
Compiled by: John Sandham IEng MIIE MIHEEM






