 Parkinson's disease (PD) is a disabling chronic neurodegenerative disorder clinically characterised by akinesia (loss or impairment of the power of voluntary movement), tremor, rigidity, and postural instability, caused mainly by brain neuron degeneration.
Parkinson's disease (PD) is a disabling chronic neurodegenerative disorder clinically characterised by akinesia (loss or impairment of the power of voluntary movement), tremor, rigidity, and postural instability, caused mainly by brain neuron degeneration.
During the last 15 years deep brain stimulation (DBS) has been established as a highly-effective therapy for advanced Parkinson's disease (PD). Patient selection, stereotactic implantation, postoperative stimulator programming and patient care requires a multi-disciplinary team including movement disorders specialists in neurology and functional neurosurgery. Deep brain stimulation (DBS) surgery was first approved in 1997 to treat Parkinson’s disease (PD) tremor, then in 2002 for the treatment of advanced Parkinson's symptoms. More recently, in 2016, DBS surgery was approved for the earlier stages of PD — for people who have had PD for at least four years and have motor symptoms not adequately controlled with medication.
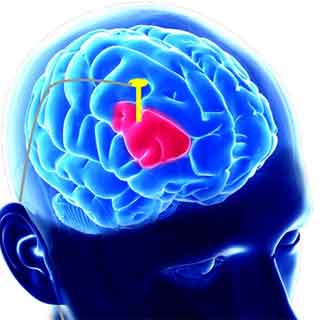
In DBS surgery, electrodes are inserted into a targeted area of the brain, using MRI (magnetic resonance imaging) and recordings of brain cell activity during the procedure. A second procedure is performed to implant an IPG, impulse generator battery (similar to a pacemaker). The IPG is placed under the collarbone or in the abdomen. The IPG provides an electrical impulse to a part of the brain involved in motor function. Those who undergo DBS surgery are given a controller to turn the device on or off.
DBS is most effective for people who experience disabling tremors, wearing-off spells and medication-induced dyskinesias (abnormality or impairment of voluntary movement), with studies showing benefits lasting at least five years. That said, it is not a cure and it does not slow PD progression. It is also not right for every person with PD. It is not thought to improve speech or swallow issues, thinking problems or gait freezing. Like all brain surgeries, DBS carries a small risk of infection, stroke, bleeding or seizures. DBS surgery may be associated with reduced clarity of speech. A small number of people with PD have experienced cognitive decline after DBS surgery. It is important that a person with PD considering DBS surgery be informed about the procedure and be realistic in his or her expectations.
Surgical procedures are used to treat a variety of disabling neurological symptoms — most commonly the debilitating symptoms of Parkinson’s, such as tremor, rigidity, stiffness, slowed movement and slowed walking. Also used to treat essential tremor, a common neurological movement disorder. It does not damage healthy brain tissue or destroy nerve cells. Instead, the procedure interrupts problematic electrical signals from targeted areas in the brain. At present, the procedure is used only for patients whose symptoms cannot be adequately controlled with medications.
The procedure uses a surgically implanted, battery-operated medical device called a neurostimulator, similar to a heart pacemaker and approximately the size of a stopwatch, to deliver electrical stimulation to targeted areas in the brain that control movement, blocking the abnormal nerve signals that cause tremor and PD symptoms. Before the procedure, a neurosurgeon uses magnetic resonance imaging (MRI) or computed tomography (CT) scanning to identify and locate the exact target within the brain where electrical nerve signals generate the PD symptoms.
During surgery, some surgeons may use microelectrode recording which involves a small wire that monitors the activity of nerve cells in the target area to more specifically identify the precise brain target that will be stimulated.
Generally, these targets are the thalamus, subthalamic nucleus (STN) and a portion of the globus pallidus. Once the system is in place, electrical impulses are sent from the neurostimulator up along the extension wire and the active contacts of the lead in the brain. These impulses interfere with and block the electrical signals that cause PD symptoms.
The DBS System Consists of Three Components
The lead (also called an electrode) is a thin, insulated wire inserted through a small opening in the skull and implanted in the brain. The tip of the electrode is positioned within the targeted brain area. The extension is an insulated wire passed under the skin of the head, neck and shoulder, connecting the lead to the neurostimulator. The neurostimulator (the battery pack") is the third component and is usually implanted under the skin near the collarbone. In some cases, it may be implanted lower in the chest or under the skin over the abdomen.
Prognosis
Although most people still need to take medication after undergoing DBS, many people experience considerable reduction of their PD symptoms and can greatly reduce their medications. The amount of reduction varies from person to person. The reduction in dose of medication leads to decreased risk of side effects such as dyskinesia (involuntary movements of the arms, legs and head). There is a one to three percent chance of infection, stroke, cranial bleeding or other complications associated with anaesthesia, per side that is done.
Compared with surgical lesioning procedures, chronic DBS used with the standard stimulation parameters for PD leads to no, or only minimal, tissue damage and is therefore largely reversible. Furthermore, unlike lesioning, bilateral DBS can be implemented without significantly increasing side effects. It is possible to adjust stimulation parameters postoperatively and in the course of the disease. In different randomised controlled trials DBS showed a better functional outcome with fewer side effects and therefore almost completely replaced lesional surgery in industrialised nations.
‘BrainSense’ Technology from Medtronic
 In 2020 Medtronic obtained the European CE Mark for its Percept PC neurostimulator that uses smart feedback technology to deliver deep brain stimulation to treat Parkinson’s and other neurologic diseases. The so-called BrainSense technology powering the Percept PC can record brain signals at the same time that it delivers therapy. It is compatible with full-body MRI scans in scanners up to 3 Tesla, allowing patients to continue to receive necessary imaging. The implant has a longer battery capacity than Medtronic’s Activa PC deep brain neurostimulator while having a smaller body.
In 2020 Medtronic obtained the European CE Mark for its Percept PC neurostimulator that uses smart feedback technology to deliver deep brain stimulation to treat Parkinson’s and other neurologic diseases. The so-called BrainSense technology powering the Percept PC can record brain signals at the same time that it delivers therapy. It is compatible with full-body MRI scans in scanners up to 3 Tesla, allowing patients to continue to receive necessary imaging. The implant has a longer battery capacity than Medtronic’s Activa PC deep brain neurostimulator while having a smaller body.
A short pulse width lets the device provide a wider array of stimulation capabilities, which physicians can take advantage of to achieve optimal results. Moreover, the potential to upgrade the system will be useful in the future when expanded capabilities are introduced by Medtronic via a simple software update.
“DBS is proven to significantly improve motor function in people with Parkinson’s disease compared to standard medication alone – but with currently-available systems, physicians need to make therapeutic decisions mostly based on clinical assessments and patient-reported information,” said Professor Andrea Kühn, head of Movement Disorders and Neuromodulation, Charité University Hospital, Berlin, in a Medtronic announcement. “Percept PC with BrainSense technology is a game changer. Patients and their care teams will have objective patient-specific brain signal data – including data recorded outside the clinic in patients’ everyday lives. With this technology, doctors could tailor therapy more precisely to the individual needs of each patient based on data from neuronal activity.”
Sources:
https://www.parkinsons.org.uk/information-and-support/deep-brain-stimulation
https://www.medtronic.com/uk-en/c/dbs-percept-pc.html
https://www.michaeljfox.org/news/deep-brain-stimulation
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3002606/
https://www.parkinson.org/Understanding-Parkinsons/Treatment/Surgical-Treatment-Options/Deep-Brain-Stimulation
Edited by John Sandham.