Part 1
Photobiomodulation (PBM)
 Photobiomodulation (PBM) also known as low-level level laser therapy is the use of red and near-infrared light to stimulate healing, relieve pain, and reduce inflammation. The primary chromophores have been identified as cytochrome c oxidase in mitochondria, and calcium ion channels (possibly mediated by light absorption by opsins). Secondary effects of photon absorption include increases in ATP (Adenosine diphosphate (ADP) is an important organic compound in metabolism and is essential to the flow of energy in living cells), a brief burst of reactive oxygen species (ROS), an increase in nitric oxide, and modulation of calcium levels.
Photobiomodulation (PBM) also known as low-level level laser therapy is the use of red and near-infrared light to stimulate healing, relieve pain, and reduce inflammation. The primary chromophores have been identified as cytochrome c oxidase in mitochondria, and calcium ion channels (possibly mediated by light absorption by opsins). Secondary effects of photon absorption include increases in ATP (Adenosine diphosphate (ADP) is an important organic compound in metabolism and is essential to the flow of energy in living cells), a brief burst of reactive oxygen species (ROS), an increase in nitric oxide, and modulation of calcium levels.
Tertiary effects include activation of a wide range of transcription factors leading to improved cell survival, increased proliferation and migration, and new protein synthesis. There is a pronounced biphasic dose response whereby low levels of light have stimulating effects, while high levels of light have inhibitory effects. It has been found that PBM can produce ROS in normal cells, but when used in oxidatively stressed cells or in animal models of disease, ROS levels are lowered. PBM is able to up-regulate anti-oxidant defences and reduce oxidative stress. It was shown that PBM can activate NF-kB in normal quiescent cells, however in activated inflammatory cells, inflammatory markers were decreased. One of the most reproducible effects of PBM is an overall reduction in inflammation, which is particularly important for disorders of the joints, traumatic injuries, lung disorders, and in the brain.
PBM for neurocognitive impairments
The Quietmind Foundation, a not-for-profit clinical research, consultation, and training organisation at the forefront of non-invasive, drug-free treatments for dementia and other neurocognitive impairments, has completed the first-ever clinical trial of its type to assess a new approach to improve mental functioning for sufferers of early-stage dementia. Infrared light therapy could potentially be used to help people living with dementia. A pilot study used a helmet to beam the light into healthy volunteers' brains and found improvements in their memory, motor function, and processing skills.
Alzheimer’s disease (AD) is a common, chronic expensive debilitating neurodegenerative disease with no current treatments to prevent the physical deterioration of the brain and the consequent cognitive deficits. The current pathophysiology of Alzheimer’s disease is the accumulation of neurofibrillary tangles (NFTs) of hyperphosphorylated tau protein and amyloid-beta (Aβ) plaques. Antibody therapy of Tau and Amyloid beta, vaccines and other methods to decrease Tau and or Amyloid have not been successful after considerable pharmaceutical and biotech efforts.
Recent animal models used photobiomodulation (PBM) with near infrared light to treat mice with NIR, 20 times over a four-week period; the NIR treatment (600–1000 nm wavelength) was associated with a reduction in the size and number of amyloid-β plaques in the neocortex and hippocampus.

A small pilot double blind, placebo-controlled trial (n=11) 6 active, 3 controls and 2 dropouts assessing the effect of 28 consecutive, six-minute transcranial sessions of near infrared (NIR) stimulation using 1060–1080 nm light emitting diodes. Subjects were independently diagnosed with dementia conducted in an outpatient behavioural healthcare clinic. IRB approval was obtained through the Quietmind Foundation’s institutional review Board (IRB). Results showed changes in executive functioning; clock drawing, immediate recall, praxis memory, visual attention and task switching (Trails A&B) as well as a trend of improved EEG amplitude and connectivity measures. Neuroplasticity has also been reported with NIR light stimulation and mitochondrial enhancement.
Alzheimer’s disease (AD) is a common, chronic progressive, expensive neurodegenerative disorder slowly resulting in dementia. Its etiology and pathogenesis is complex, with many genetic and environmental risk factors including stress and insulin resistance. The expression of many genes, and upregulation of multiple pathogenic pathways result in amyloid β peptide (Aβ) deposition, tau hyperphosphorylation, inflammation, reactive oxidative stress (ROS), mitochondrial disorders, insulin resistance, methylation defects and down regulation of neuroprotective factors. Antibody therapy of Tau and Amyloid beta, vaccines and other methods to decrease Tau and or Amyloid have not been successful after considerable pharmaceutical and biotech efforts.
For example, Eli Lilly in Indianapolis announced a major change to its closely watched clinical trial for the Alzheimer’s drug solanezumab which failed to reach statistical significance. “A major challenge of such trials is how to measure the drug’s benefits,” says Dennis Selkoe, a Neurologist at Brigham and Women’s Hospital in Boston, who is not involved in the Lilly trial. “Although people with early Alzheimer’s may show mild memory impairment and problems with attention and focus, they can often follow recipes, make a cup of coffee, or drive a car,” Selkoe reiterates. Such abilities are unlikely to change much over the course of an 18-month clinical trial.”
The role of emerging pathogens such as dental spirochetes, Borrelia Bd, with bacterial biofilms and is also gaining traction. Fungal and even viral infections have been implicated. Infections induce potent immune responses, too, and they likely worsen the problem says Rudy Tanzi. “Normally, brain immune cells called microglia clear amyloid proteins from the brain. But when these cells get fired up in response to infection, they stop, causing the proteins to build up even faster.” Tanzi’s team at Harvard showed in a 2014 Nature paper, that the amyloid proteins that fill up the brain then spark the creation of tau tangles, which cause more brain cell death. Mitochondrial dysfunction has important roles in the neurodegenerative cascade. Amyloid β can interact with the mitochondria and cause mitochondrial dysfunction.
Finally, misfolded proteins of Tau and Amyloid β by proteasomes have been elucidated in AD. Therefore, it has been proposed that targeting the mitochondria, increasing ATP in proteasomes for ubiquitination of misfolded proteins, decreasing inflammation and even antibacterial and anti-viral effect of NIR light could prove valuable for AD therapeutics and is a safe, simple and effective approach to treat early to mid-dementia in Alzheimer’s and other related neurodegenerative disorders.
The study objective was to determine if intensive near-infrared treatment (INIRT) using 1072 nm IR will affect significant positive changes in mood, behaviour, and cognitive functioning of people with dementing illness.
Neuroplastic effects of transcranial NIR stimulation (tNIRS) as a tool on the motor cortex to modulate cortical excitability in the corticospinal pathway and intracortical circuits was recently published by Chaleb and his lab at the University of Bonn. They used tNIRS at wavelength of 810 nm for 10 min over the hand area of the primary cortex and transcranial magnetic stim at 2.2 Tesla to assess levels of magnetic evoked motor-evoked- potentials of the dorsal interosseous in human brains in 55 healthy volunteers. They concluded that tNIRS is suitable as a tool for influencing cortical excitability and activity.
Study hypothesis: The provision of brief, repeated exposure to 1072 nm infrared stimulation of the cortex surface improves cognitive and behavioural functioning as indicated by normalization of EEG activity, increased cerebral oxygenation, and demonstrated improvement on standardised neuropsychological measures.
This single-centre, double-blind, randomized, placebo-controlled clinical trial of the 1072 nm Infrared Light Stimulation Helmet was analysed using:
- Patients recruited from several local continuing care communities and using print and online media. All subjects were independently diagnosed with probable Alzheimer’s dementia by a neurologist. Patients were excluded if there was diagnosis of multi-infarction dementia or Parkinson’s disease.
- Subject assessment: Testing included Mini Mental Status Exam MMSE, Quantitative EEG (QEEG), Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog) that were administered the first day of treatment and within 3 days of completing the required 28 consecutive exposure sessions.
- Surface cortical perfusion was measured before and after each treatment session using infrared spectroscopy. A two-minute baseline was recorded using the Biocomp Research Hemoencephalography recording system and Bioexplorer software.
Clinical applications
It is thought that the actions of NIR light shone on the head and penetrating into the brain are multi-factorial, but one clear effect is the anti-inflammatory action of transcranial PBM. The versatile benefits of PBM on the brain and the central nervous system, encourages further study of its ability to reduce neuroinflammation. Recent clinical brain PBM therapy studies have been focused on conditions such as AD, PD, TBI and ischemic stroke, as well as MDD. However there is also a growing interest for application of this non-invasive modality in perfectly healthy individuals to improve their cognitive abilities (cognitive enhancement). Despite the existence of several animal studies, there have only been a few studies on the efficacy of PBM therapy in AD and dementia patients. Regarding these human studies, significant improvements in sleep quality, mood states, EEG patterns as well as improved cognitive function including memory and attention, have been obtained as a consequence of NIR PBM therapy.
Parkinson’s disease: To date, the majority of the clinical investigations revealed positive impacts of transcranial PBM therapy in conditions such as TBI, stroke and depression, in which the target area was in the cortical regions of the brain. On the other hand, PD pathogenesis is linked to abnormalities in the SNc, a midbrain structure that is located at a depth 80–100 mm from the coronal suture, below the dura. Studies have suggested that light in the NIR region may not penetrate the human brain deeper than 20 mm from the cortical surface. This is considered to be a clear limitation in the application of transcranial PBM therapy in human PD. However, in the only (non-controlled, non-randomized) study in PD patients, improved motor and cognitive functions has been reported following 2 weeks of transcranial PBM therapy.
Part 2
The Experimental PBM Device
The experimental device used 1100 LEDs set in 15 arrays of 70 LEDs/array with all matched to 1060–1080 nm and pulsed at 10 hz with a 50% duty cycle. Stimulation was administered for 6 minutes daily over 28 consecutive days. All subjects were reassessed after the treatment cycle.
Data analysis: Cohorts consisted of active treatment group and controls (N=11). The pre/post design allows improvement or arrested decline in mood, behaviour, and cognitive functions to be established with relatively powerful statistical analyses. Repeated ‘Analysis of Variance’ measures (ANOVA’s) were used with each measure to determine overall improvement in the treatment group relative to the placebo controls. Additional categorical variables such as gender and/or covariates, such as age, were included in ANOVA’s to determine whether the overall effect is consistent across these factors. Multiple regressions were performed to determine the effects of combinations of factors on the effectiveness of the treatment, and a canonical correlation will assess how the outcome measures interact. Finally, parallel analyses were conducted with the 30 daily near-infrared cerebral oxygenation (HEG) measures. This enabled an attribution of the expected improvements in mood, behaviour, and cognitive function to the mediating variable of cerebral blood flow.
Results:
Delta power increase = improved alertness, attention. Alpha decrease = less anxiety.
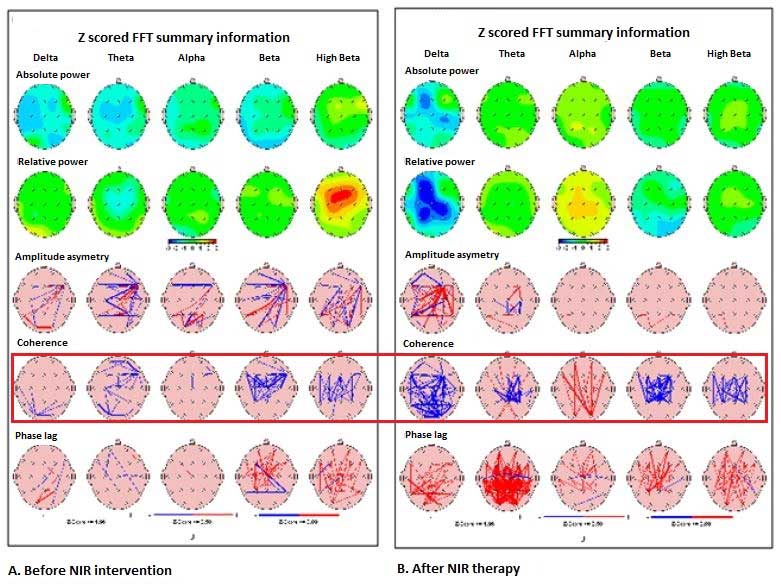
Similar QEEG improvements before and after NIR Helmet intervention was seen in the other 5 patients, but not in control patients (Figure 2 below). Quantitative EEG changes before NIR helmet and after as seen below; Green colour indicates regions where values of power were between 0 and 1SD; Yellow between 1 and 2SD; Red between 2 and 3 SD. The red box highlights pre and post-coherence summary maps.
Systemic review
A search of the world’s medical literature of significant therapy in Alzheimer’s and dementia fails to reveal any published long-term improvement to date, whether medical or by devices. NIR trials in Traumatic Brain Injury (TBI) have been published by Naesar and Hamblin. NIR light passes readily through the scalp and skull and arrive at the cortical surface of the human brain. The primary photoreceptors for NIR light and red light are mitochondria. Cortical neurons are rich in mitochondria with increased biochemical pathways such as increased ATP and signalling pathways activated by ROS. Photobiomodulation (PBM) is based upon the ability of the light to alter cell metabolism as it is absorbed by general haemoproteins and cytochrome c oxidase (COX) in particular.
Naesar reported eleven chronic TBI patients whose cognition improved following treatment with red and NIR light emitting diodes (LEDs) applied transcranially to forehead and scalp at 10 minutes per area and red light nasally with 18 outpatient sessions. Neuropsychological testing at 1, 2 and after 18 treatments of LED treatments demonstrated improvement in the Stroop test for Executive Function.
Interpretation
This is the first published report that has shown a trend of improvement in executive functioning in patients with MCI in Alzheimer’s and dementia with NIR 1072 nm light. Even though this pilot control study was very small, n=11 with 8 patients treated with NIR helmet for 28 days and 3 placebo control and 2 dropouts which often occurs in Alzheimer’s clinical trials, there was some trend of improvement in the MMSE (especially clock drawing, ACOG (especially on attention and digit span forward-verbal recall), and Quantitative QEEG; in other words, executive functioning, our objective.
Any statistical analysis of a small pilot study in MCI patients is problematic when n=11, 6 treated who completed the study, 3 placebo controls and 2 dropouts; the most revealing was to graph the result of the data of each ADAS Cog 11 and the clock drawing. The value of clock drawing has been found to be moderately sensitive and specific for detecting executive cognitive dysfunction in people even with normal MMSE. Although this was a small placebo controlled pilot study of PBT in which there was no statistical improvement in treated patients over controls, 28 days is a very short period of time in Alzheimer’s and dementia time line. Quietmind Foundation has had experience now in several Alzheimer’s patients treated with PBM adding Neurobiofeedback (NBB) with marked improvement in executive function and memory using a two year treatment protocol. Patients are being observed over 4–5 years who are showing continued cognitive and functional improvement with no medications other than vitamins and supplements for improve gut flora and chelation of heavy metals and other neurotoxins. Furthermore the neuroplastic effects of transcranial stimulation on brain motor cortex in combination with TCMS provided tools for studies of human cortical neuroplasticity. Recent human and animal studies have demonstrated the NIR light applied over the cortex has beneficial effect on stroke rehabilitation and may minimize the cognitive deficits in traumatic brain injury.
Conclusion
Because neural tissues contain large amounts of mitochondrial CCO, application of red to NIR lights for brain PBM therapy is highly attractive. The main problem so far has been getting enough light into the brain to accomplish the beneficial effects. In recent years, irradiation in the wavelength range between 980 nm and 1100 nm has been growing rapidly, and its different mechanisms of action including stimulation of ion channels and water molecules suggest it might even be combined with red/NIR. Improving cerebral metabolic function, stimulating neurogenesis and synaptogenesis, regulating neurotransmitters, and providing neuroprotection via anti-inflammatory and antioxidant biological signalling are the most important effects of brain PBM therapy. The overall results from extensive preclinical and clinical studies in the brain PBM field suggest that modest levels of red and NIR light show biostimulatory effects without any thermal damage, and could improve neurobehavioral deficits associated with many brain disorders. Nevertheless, it is still not completely clear whether chronic repetition of brain PBM will be necessary for sustained clinical benefit, especially in psychological and neurodegenerative disorders. Owing to the beneficial impacts of brain PBM therapy in depression and anxiety, new trials for other psychiatric disorders such as schizophrenia, autism, bipolar, attention-deficit hyperactivity and obsessive–compulsive disorders might well emerge in the future. Development of new techniques for effective light delivery to deeper structures of the brain is crucial, because of involvement of the limbic system and midbrain abnormalities seen in some brain disorders. In this respect, intracranial and intranasal irradiation methods, as well as the oral cavity route, even via the ear canal could be options. Although therapeutic influences of intracranial PBM therapy has been focused on PD researches, it is postulated that developing this technique also potentially effective for those conditions that are associated with limbic system dysfunctions such as anhedonia, anxiety as well as impaired emotional processing. Preliminary evidence of benefit has been obtained in autism spectrum disorders. There is an epidemic of AD that is expected to hit the Western world as the overall population ages, and there has been a noticeable lack of any effective pharmacological therapies that have been approved for AD. Although the evidence for the effectiveness of PBM in the treatment of AD is still very preliminary, it is possible that PBM will play an even larger role in society in years to come if clinical trials now being conducted are successful. The authors conclude that clinic or home-based PBM therapy using laser or LED devices will become one of the most promising strategies for neurorehabilitation in upcoming years.
The potential effect of Photobiomodulation on Alzheimer’s dementia with results suggestive a trend of improvement in executive functioning; clock drawing, immediate recall, praxis memory, visual attention and task switching (Trails A&B) as well as improved EEG amplitude and connectivity measures. Although this very small pilot clinic study using low level light at 1070 nm to increase ATP in the mitochondria and proteasomes and induce neural plasticity did not reach statistical significance, the short duration of therapy and small number of participants should not defer further clinical trials. Recently a large clinical trial of monthly IV Aducanumab for early Alzheimer’s disease at 6 months found that at higher the dose of drug, the less people with Alzheimer’s declined on tests of memory and thinking skills. Headaches in some patients and brain scans some brain swelling was detected in those taking the higher doses. PBM and NBB have virtually no side effects.
The addition of PBM and NBB in combination with novel medications in Alzheimer’s may be another potential therapeutic strategy.
Sources:
https://www.quietmindfdn.org/trials-708691.html
https://www.sciencedirect.com/science/article/pii/B9780128037263000043
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5459322/
https://www.bmedreport.com/archives/23655
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523874/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6041198/


