Up until the mid 1980s, The NHS had traditionally focussed on medical equipment maintenance (excluding procurement planning, user training, and quality records). It was during the 80s that staff in the NHS realised that there was a need for better management of maintenance, training, and procurement.
The key to all of this was quality management. In the 1990s The Medical Devices Agency produced a document entitled DB 9801 - Medical Devices and Equipment Management for Hospital and Community-Based Organisations. This document was detailed, giving descriptions of all areas that impacted on medical devices management within an organisation.
Since this document was produced, NHS Hospitals have been writing medical equipment policies that incorporate the guidance from this document. Unfortunately the majority of NHS Hospitals have not been able to implement their policies and do not maintain quality at the levels that their policies aspire to.
Where do medical equipment management standards come from?

- In the 1950's there were no Electronic and Biomedical engineering departments (EBME) in the National Health Service (NHS).
- In the 1960's, the Department of Health and Social Security published Hospital Technical Memorandum number 8 called "safety code for electro-medical apparatus". The purpose of the document was to establish adequate standards for the design and construction of electro-medical apparatus since no other relevant national standard existed at the time.
- By the 70's some NHS Hospitals had started thinking about maintenance and management of medical equipment. The NHS produced guidance in 1977 that was not implemented in the NHS but did raise the profile of medical engineering. In 1979, HTM 8 was superseded by the British Standard BS 5724 part 1. This document is a comprehensive specification for safety of medical electrical equipment.
- In the 80's the DHSS issued HEI 95 entitled "Code of practice for acceptance testing of medical electrical equipment". BS 5724 part 1 was also revised, making it identical to the International Electro-technical Commission standard IEC 601-1: 1988. The standard was subsequently re-numbered as IEC 60601-1.
- In the 90'sThe HEI 95 document was officially withdrawn on the publication by the Medical Devices Agency of MDA DB9801 - Medical device and equipment management for Hospital and community based organisations. This document described a more strategic management approach to delivering EBME services.
- In November 2006, the MHRA published Device Bulletin DB2006 (05) - "Managing Medical Devices - Guidance for healthcare and social services organisations". http://www.ebme.co.uk/files/upload/stds/Managing_Medical_Devices_DB2006(05).pdf
The risk of not managing medical equipment correctly has been recognised by the NHS litigation authority (NHSLA), and also by the care quality commission (CQC). As a result of the perceived risks both of these organisations have included audit criteria that NHS Hospitals must abide by if they are to be successful when these audits are carried out.
After being involved with the implementation of these standards across multiple organisations it has become apparent to me that the reason why the NHSLA and CQC are auditing against these criteria is because the perceived risk is real, and patients are dying and being seriously injured because many Hospitals do not successfully implement the requirements of the standards and regulations (usually stated within their policies).
How to implement?
In my experience, many organisations are able to produce reasonable policies by sharing knowledge with other organisations (in other words, they copy the Hospital's policies). Although sharing of documents is a good idea, this in itself does not mean the Hospital will be successful in implementing the standards. To implement the standards the Hospital must take responsibility for producing and managing processes that relates to the policy. Once these processes have been agreed, those processes must then be facilitated by the staff responsible. To enable the facilitation, it is best to set up project leads (usually at least two people for an average acute Hospital) one of these people will be at a senior management grade (medical devices manager), and the other will act as an assistant. There are key stakeholders within an organisation that need to be on the medical equipment steering group-this group will be accountable for the medical equipment policy. The medical devices manager may chair the medical equipment steering group, which will have representation from all clinical and non-clinical directorates.
Procurement is very important to ensuring standardisation, reduction in risk, and cost control (or cost savings). The training and development department are important in ensuring that users are trained and understand the risks involved with the equipment they use. The medical equipment maintenance department are important because they ensure that equipment is kept in an operational and safe condition. The Finance team should have a representative on the steering group to ensure that budgets are adjusted where necessary. This group will usually have at least one senior executive (usually the board level executive responsible for medical devices) that can direct the steering group from a corporate perspective.
DB2006 (05) Managing Medical Devices: Guidance for healthcare and social services organisations
This document updates and replaces previous guidelines published in DB 9801 'Medical device and equipment management for Hospital and community-based organisations' (including supplement 1 'Checks and tests for newly-delivered medical devices' and supplement 2 'Guidance on the sale, transfer of ownership and disposal of used medical devices') and also DB 2000(02) 'Medical devices and equipment management: repair and maintenance provision'.
It is intended primarily for people in Hospital and community based organisations (including social services) that are responsible for the management of medical devices, to help them set up systems that minimise risks associated with the use of those medical devices.
The purpose of this document is to outline a systematic approach to the purchasing, deployment, maintenance, repair and disposal of medical devices.
This guidance aims to:
- provide balanced information for groups developing local policy
- identify relevant legislation
- address the strategies for ownership and use of medical devices
- help healthcare organisations meet the Healthcare Commission's core standards for the safe use of medical devices
- Identify sources of additional guidance.
The main topics covered are:
- monitoring/audit
- reporting adverse incidents
- acquiring the most appropriate device
- acceptance procedures for newly delivered devices
- maintenance and repair
- training
- adequacy of manufacturer instructions
- prescribing the best device
- decontamination
- decommissioning
- disposal
- transfer of ownership
- legal liabilities
It also states "Responsible organisations should appoint a director or board member with overall responsibility for medical device management" There should be clear lines of accountability throughout the organisation leading to the board. These lines of accountability should be extended, where appropriate, to include general practitioners, residential and care homes, community based services independent Hospitals providing services for NHS patients, managed care providers, PFI organisations and other independent contractors. It is important to establish who is accountable, and where there is a need for joint accountability arrangements".
The board should ensure that policies address:
- Decontamination
- equipment life cycle
- procurement
- records
- adverse incident reporting
- actions required on MHRA's Medical Device Alerts and manufacturers' corrective notices
- training
- technical specifications
- regulatory compliance and related issues
- rationalisation to single models, where possible
- risk management
- equipment inventory
- manufacturer's instructions
- Disposal.
In the past few decades some NHS Hospitals have been involved in producing these standards, and are therefore up to date, and others have been given documentary advice through the NHS executive, National Audit Office [NAO], Medicines and Healthcare Products Regulatory Agency [MHRA] (previously the Medical Devices Agency - MDA), and asked to implement the recommendations without management training or external assistance.
Many NHS organisations have not funded the increased responsibilities thereby leaving their medical equipment management services struggling to keep up with the latest NHS executive and Dept of Health initiatives.
This has led to some services still operating with the same number of staff that they had in the 80's, but with a much larger inventory of medical equipment to manage and maintain, and increased expectations from the Care Quality Commission (CQC) and the National Health Service Litigation Authority. Unfortunately, many of the top executives have little or no understanding of the complexity of meeting the standards
The National Audit Office produced a report on The Management of Medical Equipment in NHS Acute Trusts in England.
See: http://www.nao.org.uk/publications/nao_reports/9899475.pdf
The report concludes that, although there are examples of good practice, overall more needs to be done by NHS Trusts to allocate clear responsibility for medical equipment at board level. Trusts need to ensure that inventory information is comprehensive and used fully in decision making. (This inventory information should include diagnostic imaging equipment, pathology equipment, and general biomedical equipment) Procurement of medical equipment needs to be better co-ordinated across Trusts, with more involvement of medical technical personnel. They can also usefully contribute to non-clinical aspects of user training. We also recommend action that should help to improve the standards of reporting of adverse safety incidents, and to reduce their occurrence.
The risks of a Hospital failing to implement standards and regulations:
- Patients may die or be seriously harmed
- Longer bed stays / bed management problems
- Higher costs for capital purchase
- Higher costs for revenue purchases
- Higher costs for externally contracted maintenance
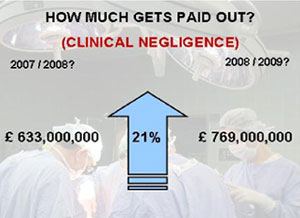
- Higher legal fees (Clinical negligence)
- Higher NHSLA costs
- Failure to meet CQC and NHSLA management and quality standards
The actual financial cost of doing nothing... (i.e. not investing in medical equipment management) is higher than funding the positions needed to have a Hospital that meets the current standards from the CQC and the NHSLA.
Medical equipment managers will hold a senior management post, but will be expected to:
- Keep up with the latest innovations
- Advise on all aspects of medical devices management in line with National Audit office and MHRA recommendations.
- Understand clinical interactions between device users, and patients.
- Manage larger multi-disciplinary teams
- Advise Trust executives / attend senior management / executive meetings.
- Liaise with other senior managers in procurement, governance, finance, infection control, etc.
- Patient Care is compromised and costs are escalated.
Additional resources:
A medical equipment manager in is expected to be able understand and deliver:
- Planned equipment replacement programmes Tender advice to head of procurement
- MHRA liaison (Incident investigation) Advising medical and nursing directors on serious untoward incidents (SUI's)
- NHSLA advice and reporting for medical devices governance
- CQC advice and reporting
- Policy production and implementation
- Evaluating devices
http://www.ebme.co.uk/articles/management/321-ebme-medical-device-evaluation - Commissioning devices
http://www.ebme.co.uk/articles/management/105-acceptance-testing-of-medical-equipment/ - Planned maintenance
http://www.ebme.co.uk/articles/management/325-planning-maintenanceindex.php
http://www.ebme.co.uk/articles/management/327-reliability-centred-maintenance-rcm - Remedial Maintenance
http://www.ebme.co.uk/articles/maintenance - User training
- Technical training
- Database reporting and management
- Income generation through service level agreements with other Hospitals.
http://www.ebme.co.uk/articles/management/328-service-level-agreements-sla-s-and-customer-satisfaction - Service contract assessment and control
http://www.ebme.co.uk/articles/management/313-ebme-service-maintenance-contract-evaluation-process - Cost saving improvements
http://www.ebme.co.uk/articles/management/314-cost-saving-improvementsindex.php - Equipment library set up and management http://www.ebme.co.uk/articles/management/320-making-the-case-for-an-equipment-libraryindex.php
- Gas safety
- Electrical safety http://www.ebme.co.uk/articles/electrical-safety
- Technician training
http://www.ebme.co.uk/articles/clinical-engineering - Manufacturers and in-house training
- Disposal WEE regulations
- Quality management systems
http://www.ebme.co.uk/articles/management/316-ebme-management
http://www.ebme.co.uk/articles/ebme-quality-management
Author: John Sandham IEng MIHEEM MIET November 2009








