The current state of device procurement does not easily allow for equipment standardisation that can reduce costs and risks.
Hospitals face fundamental questions of patient safety, care, and budgetary concerns when buying, using, or maintaining medical equipment. In this regard, it is imperative to improve safety whilst reducing costs. There has been increasing recognition that there is a serious issue in respect of medical devices management covering the areas of procurement, training, maintenance, and governance.
Medical Devices internal policy questions 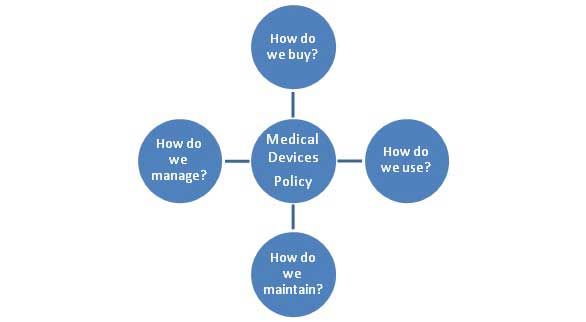
This medical equipment issue, researched and documented by the National Audit Office, National Patient Safety Agency, Medicines and Healthcare Products Regulatory Agency, National Health Service Litigation Authority, and World Health Organisation, impacts on healthcare costs and patient safety. This issue has led to revisions for the Health and Social Care Act Regulations in the UK, which are enforced by the Care Quality Commission.
Hospitals must ensure that patients have access to 'up to date' medical technology to improve healthcare outcomes. To achieve this, the Hospital management must set policy to guide organisational conduct relating to acquisition, user training, maintenance and governance.
Healthcare technology must be managed to ensure technology procurement decisions are right for the patient and economically sustainable for the organisation. Improving technology management ultimately improves care and reduces organisational costs.
This is supported by World Health Organisation research. Hospitals must ensure a safe and sustainable way of using technology to improve patient care when providing services related to the procurement, training, and maintenance of medical technology for.
One of the World Health Organisation's strategic objectives is to ensure improved access, quality and use of medical products and technologies. This objective, together with a World Health Assembly resolution, forms the basis for establishing the global initiative on health technologies, with funding from the Bill and Melinda Gates Foundation.
The World Health Organisation had two specific objectives in mind:
- to challenge the international community to establish a framework for the development of National essential health technology programs that would have a positive impact on the burden of disease and ensure effective use of resources;
- to challenge the business and scientific communities to identify and adapt innovative technologies that can have a significant impact on public health.
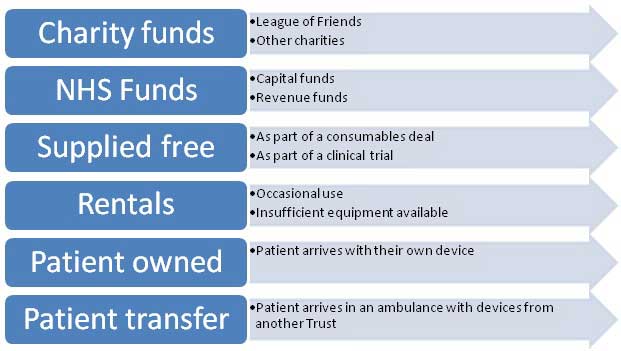
Multiple routes of acquisition
There are multiple sources of funding as illustrated below, but there are also other routes for devices to 'appear' in the Hospital. These include donations from charities, and personal donations from patients. It is not uncommon for charities and patients to buy equipment for wards and departments as gifts. This is well-meaning, but not necessarily beneficial unless the equipment is introduced as part of a planned process following the conduct described in the Medical Devices Management policy.
Device acquisition routes into the Hospital
Researching the four interrelated areas of procurement, training, maintenance, and governance has resulted in changes to Hospital policy, especially around procurement, subsequently leading to improvements in standardisation, safety and reduction in costs.
Technology group Life Cycle 
It is important to implement best practice for medical devices management within a governance framework that meets the needs of the external regulators, and the management of the organisation. More specifically, the use, maintenance, and governance of medical equipment are reliant on a central issue, namely procurement.
Planned procurement of medical equipment is making training, maintenance, and governance easier to achieve, thereby reducing risk, and cost. Moreover, this medical equipment best practice management model is improving practice which assists in patient safety and meeting budgets.
The best practice model

Users of equipment at the Hospital have a great degree of choice when buying devices. The use, maintenance, and governance of medical equipment are all reliant on a central issue, namely procurement practice. Procurement conduct must be redefined within Hospital policy, and when fully implemented, will make training, maintenance, and governance more achievable, thereby reducing risk, and cost.
Author - John Sandham - 29/10/2013
World Health Organisation. (2011). Development of Medical Devices Policies. Switzerland: WHO.