 Proton therapy is one of the most advanced treatments available to cancer patients.
Proton therapy is one of the most advanced treatments available to cancer patients.
Proton therapy is a type of particle therapy that uses a beam of protons to irradiate diseased tissue, most often in the treatment of cancer. The chief advantage of proton therapy over other types of external beam radiotherapy is that as a charged particle the dose is deposited over a narrow range of depth, and there is minimal entry, exit, or scattered radiation dose.
Proton therapy treats tumours by directing the radiation into the tumour site where doses of radiation destroy cancerous cells. Physicians can control the timing and dosage of energy from protons, which allows the maximum energy deposited directly into the tumour, reducing damage to nearby healthy tissue and thus limiting negative side effects. Protons are more precise than traditional radiation therapy, with the proton beam being fine-tuned within millimetres of accuracy to deliver maximum energy within a controlled range of the tumour.
Proton beam therapy (PBT) is safer and has significantly reduced side effects compared to conventional X-ray radiation therapy. As shown in the figure 1 below, the PBT machine accelerates protons from a hydrogen atom up to 70% of the speed of light and then aiming the beam at the tumour in the body to destroy it. It is done with a much higher precision than conventional therapy, minimising damage to the surrounding tissue and reducing exposure to radiation.

Proton therapy uses a particle accelerator to target a tumour with a beam of protons. These charged particles damage the DNA of cells, ultimately killing them by stopping their reproduction. Cancerous cells are particularly vulnerable to attacks on DNA because of their high rate of division and their reduced abilities to repair DNA damage. Some cancers with specific defects in DNA repair may be more sensitive to proton radiation.
Because of their relatively large mass, protons have little lateral side scatter in the tissue; the beam does not broaden much, but stays focused on the tumour shape, and delivers only low-dose side effects to surrounding tissue. All protons of a given energy have a certain penetration range; very few protons penetrate beyond that distance. Furthermore, the dose delivered to tissue is maximized only over the last few millimetres of the particle’s range; this maximum is called the Bragg peak, often referred to as the spread-out Bragg peak (SOBP), as shown in figure 2 below.
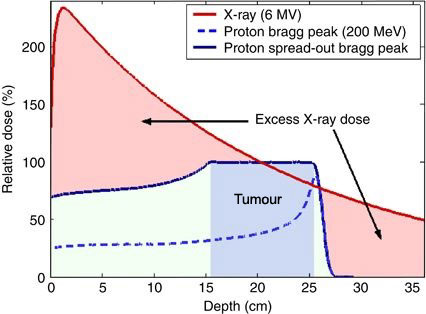
To treat tumours at greater depths, the proton accelerator must produce a beam with higher energy, typically given in eV or electron volts. Accelerators used for proton therapy typically produce protons with energies in the range of 70 to 250 MeV. Adjusting proton energy during the treatment maximizes the cell damage the proton beam causes within the tumour. Tissue closer to the surface of the body than the tumour receives reduced radiation, and therefore reduced damage. Tissues deeper in the body receive very few protons, so the dosage becomes immeasurably small.
In most treatments, protons of different energies with Bragg peaks at different depths are applied to treat the entire tumour. The total radiation dosage of the protons is called the spread-out Bragg peak (SOBP). It is important to understand that, while tissues behind (or deeper than) the tumour receive almost no radiation from proton therapy, the tissues in front of (shallower than) the tumour receive radiation dosage based on the SOBP.
Proton therapy is far more expensive than conventional therapy, so the issue of when, and how best to apply this technology is controversial. As of 2012 there have been no controlled clinical trials that demonstrate proton beam therapy yields improved survival or other clinical outcomes compared to other types of radiation therapy.
NHS Choices has stated:
‘We cannot say with any conviction that proton beam therapy is “better” overall than radiotherapy. Some overseas clinics providing proton beam therapy heavily market their services to parents who are understandably desperate to get treatment for their children. Proton beam therapy can be very costly and it is not clear whether all children treated privately abroad are treated appropriately’.
Advantages of Proton Therapy:
1) Patients can benefit from minimal radiation exposure and damage to nearby healthy tissues and organs, and hence reduced treatment-related side effects.
2) It allows where appropriate, a higher dose to be delivered to the tumour with the potential for better control or cure.
3) It can be used to treat recurrent cancers that standard x-ray radiation therapy may not be able to.
Proton treatment can also be combined with radiation, chemotherapy and biological treatments, depending on the cancer type to provide better outcomes and less tissue damage.
Sources:
https://www.nccs.com.sg/news/medical-news/nccs-bringing-in-proton-beam-therapy-for-safer-cancer-treatment
https://williamsonsource.com/proton-therapy-for-cancer-patients-coming-to-franklin/
https://www.cleveland.com/healthfit/index.ssf/2016/05/university_hospitals_unveils_ohios_first_proton_therapy_lab_to_target_certain_cancers.html
https://en.wikipedia.org/wiki/Proton_therapy
https://media.nature.com/lw926/nature-assets/bjc/journal/v108/n6/images/bjc2013100f1.jpg
Edited by John Sandham