As at 31 March 2012, the NHSLA estimated that it had potential liabilities of £18.9 billion, of which £18.6 billion relating to clinical negligence claims. This figure represents the estimated value of all known claims which may settle or be withdrawn over future years. (NHSLA Factsheet 2 - published 2012)
The NHS is carrying significant risk in terms of patient safety and expenditure. This is a serious issue because a high percentage of high risk medical devices being used without evidence of adequate training or maintenance. This is resulting in serious injuries and deaths.
There are two core issues impacting on medical devices management policy that lead to non-compliance. Firstly, training in the safe use of medical devices, and secondly maintaining and calibrating devices. This is a serious issue, as can be seen from the numerous negative news stories.
The Times on-line stated: 'The critical research conducted by Dr Foster, a consultancy that collates independent league tables on NHS trusts, also identified 27 trusts with unusually high death rates involving the deaths of 5,000 more patients in the past year than had been expected. A CQC spot check last month had uncovered soiled mattresses, poor clinical practices, mould growing in suction machines and out-of-date medical equipment.'
According to the Health select Committee: 'The evidence, particularly that from case note reviews, both in England and internationally, indicates that the extent of medical harm is substantial, even on a conservative estimate, and that much is avoidable. International studies suggest that about 10% of all patients who are admitted to hospital suffer some form of harm. Judging how far patient safety policy has been successful requires more reliable data regarding how much harm is done to patients.
Unfortunately, neither the NPSA nor the DH was able to provide us with that. Government estimates of avoidable harm and the attendant financial costs are extrapolations from old, very limited, data; and no attempt has been made to produce reliable up-to-date figures'.
In illustration 1 - Medical equipment in the news, it can be seen that risks associated with medical devices management is a nationwide concern.
To deal with the fixed budget, the trust said savings will have to be made to pay for rising energy bills, pay increments and the increased cost of drugs and medical equipment. www.bbc.co.uk/news/uk-england-tees-15544693
The old Kent and Sussex Hospital site has been earmarked for housing and will be sold to a developer, with the funds used to buy medical equipment for the new hospital, the NHS trust said. www.bbc.co.uk/news/uk-england-kent-15511517
Too many trusts are still not responding to patient safety alerts in England, campaigners say. Alerts are issued when potentially harmful situations are identified in health settings, such as the risk of overdoses or using medical equipment. www.bbc.co.uk/news/health-12527071
High-risk medical technology has been found to be infected by computer viruses and malware, health and security experts have said. They fear that the virus infections could become so severe that a patient may end up getting harmed. www.bbc.co.uk/news/technology-19979936
NHS Procurement systems and budget management processes are set up in a way that allows managers of individual wards/depts choice to buy medical equipment, as can be seen from the budget statement example below (from the Audit Commission) showing local equipment and consumable purchase ability.
In a typical NHS Trust there can be 200 wards and depts with their own budget lines for equipment. This does not allow for easy equipment standardisation that can reduce costs and risks.

What are the risks of poor management of devices?
The proper management of medical equipment has become an acute problem in the healthcare sector, due to the multitude of devices in use, changes in technology, changes in regulatory requirements, and the need to manage all these issues.
Legal issues
The Government approved legislation for device management in 2010 that relates to providers meeting the care quality standards described in the regulations. The Department of Health is assuring legal compliance through the Care Quality Commission (CQC). The CQC is monitoring Healthcare providers to ensure they are adhering to the new legislations which relates to quality provision.
'A new law governing the way we regulate health and adult social care in England came into force on 1 October 2010. This introduced a new set of essential standards of quality and safety that all care providers must meet.' (Care Quality Commission, 2010)
The law should play an important, though not dominant role in regulating the relationships between Trusts and their various stakeholders, including patients and commercial suppliers. So, for example, there are laws specifically designed to protect patients with regard to use of medical devices, and there are laws specifically designed to ensure suppliers provides services within an agreed legal terms of reference.
There is a question as to whether the CQC understand device management issues, and are really able to get to the heart of the problem. Device management is a burning public issue because it has been now been enshrined in law through 'The Health and Social Care Act 2010' (Care Quality Commission, 2010). This Act is in an area that is politically significant, impacting on levels of risk to patients, and NHS organisational reputation. The Care Quality Commission have made medical devices management a priority (under regulation 16, outcome 11) and listed device management as one of the poorest performing areas of NHS management in its 2010 report. (Care Quality Commission, 2010)
NHS Trusts must adhere to the Care Quality Commission regulations and NHSLA standards. Over the last 30 years there have been many attempts by government and healthcare agencies to address the policy issues faced when managing medical technology.
In broad terms, these policy issues have always involved the procurement, the use, the maintenance, and the governance in accordance with regulatory standards of medical technology.
A recent HM Government report shows that the medical technology market will continue to grow year-on-year. Over the next five years, medical technology is expected to grow at 10% per year.
'The medical technology market is estimated to be worth £150-170bn worldwide with growth rates forecast at 10% per annum over the next 5-6 years and a market size approaching £300bn by 2015. This growth is driven by the ageing of the world's population and the per capita income increases in healthcare expenditure across developed countries'. (HM Government, 2011, p. 10)
The NHS is struggling to manage devices they own due to their variety and complexity, changes in regulations, and new devices coming onto the market, resulting in more demands from nurses and doctors to have access to these new devices. Government policy is produced as a result of analysis of many factors affecting patient care. This will include cost, new devices, new drugs, new techniques, and pressure from manufacturers and service providers to open up the NHS market to external providers. The demand for new technology is insatiable, and this can result in unnecessary cost, additional risk, and practical constraints of how to procure equipment, how to train staff to use the equipment, and how to maintain such a wide variety of equipment. Alongside these internal demands, are the demands of the government through the regulators to ensure that the equipment is managed to the highest standards for the well-being and safety of the patient. Although all this technology is available for patient care, the quantity and variety of devices available can introduce risks of misuse, risks of overspend, and risks were equipment is unavailable due to lack of maintenance. The government have therefore decided to regulate in order to mitigate these risks. There are many other areas which impact on the organisation's policy for managing medical devices. These are a mixture of external influences, and internal influences.
Some of the external influences are shown in Figure 2 - External Governance and Regulatory Demands below.
Figure 2. External Governance and Regulatory Demands
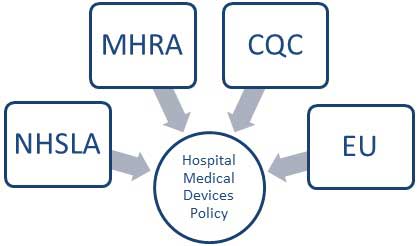
The National Health Service Litigation Authority sets the risk management standards for the National Health Service. (NHS Litigation Authority, 2012)
The Medicines and Healthcare Products Regulatory Agency regulates specific medicines and healthcare products (MHRA, Managing Medical Devices: Guidance for healthcare and social services organisations, 2006).
This guidance aims to:
- provide balanced information for groups developing local policy
The European Union regulates the manufacture of medical devices (EU Commission, 1993). These are the four key external governance and regulatory areas which influence the hospital's medical devices policy.
A 2004 project paper from the NPSA shows that many organisations were operating inefficient device management policy, as can be seen from the extract below, many organisations are still facing similar problems today.
'These audits established the following averages across the six pilot sites:
- 65% of available stock in each site was under-utilised;
- the range of infusion devices available for use was 31;
- infusion device stock was 1065;
- the cost of this stock was £1.6m.
These findings reflected an inefficient system in which infusion devices are purchased, managed and used. This is probably a national issue supported by the fact that 93 trusts initially expressed an interest in participating in this pilot work (implying that they needed help).' (National Patient Safety Agency, 2004, p. 6)
Procurement
Good procurement of medical technology can reduce the size of the inventory, reducing the value of the inventory, and thereby reduce the annual spend on replacing assets. It can also result in improved utilisation of the assets, resulting in improved outcomes for patients, and improved throughput of patients, resulting in improved revenues for the trust. The procurement policy for medical technology has an impact on the organisation in terms of cost, availability, and suitability, and strategic needs.
Uncontrolled purchasing was discussed in a National Patient Safety Agency project report (National Patient Safety Agency, 2004, p. 2)
'The project identified that uncontrolled purchasing and device management, in the absence of competency-based training, were contributing factors in causing incidents'
Training
Training is considered a high risk area by the government, and as a result of this they have introduced the regulations previously discussed. Policymakers at a government level may not understand the practical difficulties of implementing training across multiple technology groups, especially when there was limited standardisation across many groups of medical devices.
Funding training
To meet the key requirements of the regulations and standards funding is required to:
- Employ qualified staff who are able to implement the regulations and standards.
- Carry out training on the actual changes to the regulations and standards with the relevant management teams.
- Carry out training on the actual medical devices.
Staff requiring training at a Hospitals NHS Trust normally find it very difficult to leave their posts because there is insufficient funding to backfill them whilst they go through training.
For example, if 2500 staff require training on five pieces of medical equipment, each equipment training course taking one hour each, this requires 12,500 hours of replacement staff time.(There are 1000's of different equipment types in an average NHS Trust)
Maintenance
Maintenance policy for medical technology is important to the efficient running of the organisation, aiding therapeutic and diagnostic care of patients, and also to the volume of equipment required by the organisation.
Governance
Governance policy for medical technology is carried out in accordance with the requirements of the trust policy for medical devices management, which must meet the requirements of the regulators.
Serious lack of professional knowledge
There is a serious lack of professional knowledge with regard to device management, and a lack of understanding of the impact of what 'not having this knowledge' can have on the organisation, on the patient, and on them as a professional practitioners should they do something which is against the guidelines of the policy.
The importance of medical devices management cannot be understated. There have been many reported incidents where patients have been harmed or died whilst connected to medical devices. Most of these incidents could have been avoided if the professional practitioners involved had been fully aware of their responsibilities, and had been operating in accordance with the definite course of action.
 Best practice in medical devices management policy should be promoted to ensure risks to patients are minimised, and unnecessary inefficiencies in NHS organisations are avoided thereby reducing costs.
Best practice in medical devices management policy should be promoted to ensure risks to patients are minimised, and unnecessary inefficiencies in NHS organisations are avoided thereby reducing costs."This new model for healthcare has a technological basis and biomedical engineers will be the key drivers - they are the people that can deliver better utilisation and better access. They will have to learn to meet new challenges in terms of quality."
 In reality, practitioners have a difficult job to do, and are under a constant workload. Finding time to sift through policies and recognise areas of policy that impact on their professional practice is very difficult.
In reality, practitioners have a difficult job to do, and are under a constant workload. Finding time to sift through policies and recognise areas of policy that impact on their professional practice is very difficult.
Even if they do find time to sift through the policies, how do they then implement those policies unless they have sufficient support from the organisation? Policy in isolation is very ineffectual, and does not really benefit the patients, the practitioners, or the organisation. It is important to understand the reasons why policies sit on shelves gathering dust. Trusts must be more actively involved in making changes that improves their practice. Systems must be in place to ensure practitioners are able to amend their practice in accordance with changes in policy. If systems are not put in place, staff will become entrenched in practice based purely on their own knowledge and skills, and if those knowledge and skills are subsequently found to be inadequate (potentially resulting in the harm or death of a patient) as a result of the organisation not adhering to its own policy, the organisation is as much at fault as the practitioner for not providing suitable support.
The EBME Conference (www.ebmeassociates.com) on the 1st May 2013 will be addressing these issues and proposing innovative solutions based on best practice. This 4th EBME conference will be focussing on Innovations that delivers risk and cost benefits to healthcare organisations.
Author: John Sandham IEng MIET MIHEEM Jan 2013
Bibliography
EU Commission. (1993, June 14). Medical Devices Directives. Retrieved October 21, 2012, from Europa.eu:http://ec.europa.eu/enterprise/policies/european-standards/harmonised-standards/medical-devices/
MHRA. (2006). Managing Medical Devices: Guidance for healthcare and social services organisations. London: Medicines and Healthcare Products Regulatory Agency.
National Patient Safety Agency. (2004). Standardising and centralising infusion devices. London: NPSA.
NHS Litigation Authority. (2012). NHSLA Risk Management Standards 2012-13. London.