Part 1 - The need for regulation
1. Over the last 30 years there have been many attempts by government and healthcare agencies to address the issues faced when managing medical technology. In broad terms, these issues involved the procurement, the use, the maintenance, and the governance in accordance with regulatory standards of medical technology.
A recent HM Government report shows that the medical technology market will continue to grow year-on-year. Over the next five years, medical technology is expected to grow at 10% per year.
'The medical technology market is estimated to be worth £150-170bn worldwide with growth rates forecast at 10% per annum over the next 5-6 years and a market size approaching £300bn by 2015. This growth is driven by the ageing of the world's population and the per capita income increases in healthcare expenditure across developed countries' (HM Government, Strength and Opportunity, 2011, p. 10).
The NHS is struggling to manage devices they own due to the variety and complexity, changes in regulations, and new devices coming onto the market, resulting in more demands from nurses and doctors to have access to these new devices.
Figure 1 - Demands on healthcare device management policy
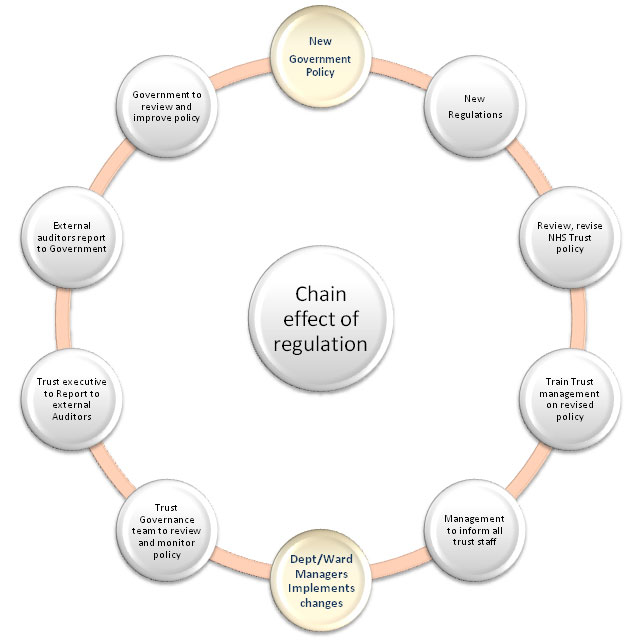
 Figure 1 indicates the internal and external demands on healthcare device management policy that will be described in more detail in this document. Government policy is produced as a result of analysis of many factors affecting patient care. This will include cost, new devices, new drugs, new techniques, and pressure from manufacturers and service providers to open up the NHS market to external providers. In figure 2 - The Chain effect of regulation, it shows the impact of new government policy on UK regulations. In 2010 the government introduced new healthcare policy which impacted on the safe use of medical technology.
Figure 1 indicates the internal and external demands on healthcare device management policy that will be described in more detail in this document. Government policy is produced as a result of analysis of many factors affecting patient care. This will include cost, new devices, new drugs, new techniques, and pressure from manufacturers and service providers to open up the NHS market to external providers. In figure 2 - The Chain effect of regulation, it shows the impact of new government policy on UK regulations. In 2010 the government introduced new healthcare policy which impacted on the safe use of medical technology.
As a result of this new policy, regulations were approved for implementation in October 2010. Care Quality Commission (CQC) Regulation 16, outcome, 11 specifically relates to the safety, and suitability, and safe use of medical devices.
Figure 2 - The chain effect of regulation
The Impact of regulation
The impact of the new regulations on 'Example' Hospitals NHS Trust resulted in a review and changes to the trust policy on medical devices management. As a result of these changes, it was necessary to then train the responsible managers on the revised policy to ensure the organisation could adhere to the new government policy. Once the managers were trained, it was necessary for them to cascade changes in policy to their teams. Department and Ward managers were expected to implement the changes. The trust governance team has a responsibility to monitor the revised policy for compliance. On a monthly basis, the governance team must report compliance levels to the 'Governance Committee'. This committee reports to the executive of the trust, and allows them to report upwards to the strategic Health Authority.
The Care Quality Commission act as the external monitor for the government to ensure regulations are adhered to and can visit any healthcare trust at any time without notice. This is meant to ensure compliance with regulations and government policy, and thereby allow the government to review and improve policy.
There are many other areas which impact on the organisation's policy for managing medical devices. These are a mixture of external influences, and internal influences. The external influences are shown in 'Figure 3 - External Governance and Regulatory Demands' below.
Figure 3 - External Governance and Regulatory Demands
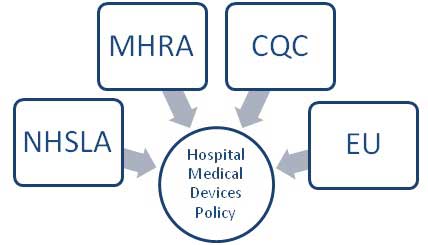 The National Health Service Litigation Authority sets the risk management standards for the National Health Service. The Medicines and Healthcare Products Regulatory Agency regulates specific medicines and healthcare products (MHRA, Managing Medical Devices: Guidance for healthcare and social services organisations, 2006). The European Union regulates the manufacture of medical devices, (EU MDD 93/42). These are the four key external governance and regulatory areas which influence the hospital's medical devices policy. If any one of these four external organisations issue new standards or regulations, 'Example' NHS Hospitals Trust must change their medical devices policy, and then implement those changes as shown in figure 2.
The National Health Service Litigation Authority sets the risk management standards for the National Health Service. The Medicines and Healthcare Products Regulatory Agency regulates specific medicines and healthcare products (MHRA, Managing Medical Devices: Guidance for healthcare and social services organisations, 2006). The European Union regulates the manufacture of medical devices, (EU MDD 93/42). These are the four key external governance and regulatory areas which influence the hospital's medical devices policy. If any one of these four external organisations issue new standards or regulations, 'Example' NHS Hospitals Trust must change their medical devices policy, and then implement those changes as shown in figure 2.
Part 2. Factors impacting on the medical devices policy